

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1


Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3
You know the right answer?
A chemistry graduate student is given 125. mL of a 1.30 M propanoic acid (HC2H, Co2) solution. Propa...
Questions in other subjects:

History, 21.12.2019 15:31

English, 21.12.2019 15:31

Mathematics, 21.12.2019 15:31

History, 21.12.2019 15:31


Mathematics, 21.12.2019 15:31


Social Studies, 21.12.2019 15:31


 is, 24.5 grams
is, 24.5 grams
 = 1.30 M
= 1.30 M .
.
 in this expression, we get:
in this expression, we get:
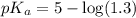

![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0513/2213/e961a.png)
![pH=pK_a+\log \frac{[KC_2H_5CO_2]}{[HC_2H_5CO_2]}](/tpl/images/0513/2213/845a9.png)
![5.02=4.89+\log (\frac{[KC_2H_5CO_2]}{1.30})](/tpl/images/0513/2213/18fd8.png)
![[KC_2H_5CO_2]=1.75M](/tpl/images/0513/2213/46461.png)





