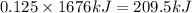Chemistry, 17.02.2020 17:27 hiplikedyani
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to form Al2O3(s) at 25°C and 1 atm. ΔHfAl2O3(s) = −1676 kJ/mol HINT: What does ΔHfAl2O3(s) mean?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1
You know the right answer?
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to fo...
Questions in other subjects:


Chemistry, 18.08.2020 22:01




Mathematics, 18.08.2020 22:01




 .
.


 moles of
moles of 
 moles of
moles of