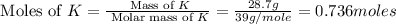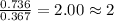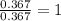Chemistry, 17.02.2020 17:18 patience233
What is the empirical formula of a compound composed of 28.7 g of potassium ( K ) and 5.87 g of oxygen ( O )? Insert subscripts as needed.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
What is the empirical formula of a compound composed of 28.7 g of potassium ( K ) and 5.87 g of oxyg...
Questions in other subjects:

English, 30.11.2021 22:20


Social Studies, 30.11.2021 22:20




Computers and Technology, 30.11.2021 22:20

Mathematics, 30.11.2021 22:20


Engineering, 30.11.2021 22:20