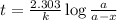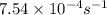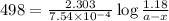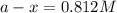Chemistry, 17.02.2020 16:38 dragaozin4505
The rate constant for a first order reaction with a single reactant is 7.54x10-4 s-1. If the initial reactant concentration is 1.18 M, what will the reactant concentration be after 8.30 minutes?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1


Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
The rate constant for a first order reaction with a single reactant is 7.54x10-4 s-1. If the initial...
Questions in other subjects:

Mathematics, 23.11.2020 15:00

English, 23.11.2020 15:00

English, 23.11.2020 15:00

History, 23.11.2020 15:00





Mathematics, 23.11.2020 15:00

English, 23.11.2020 15:00