Chemistry, 16.02.2020 19:41 queenkimm26
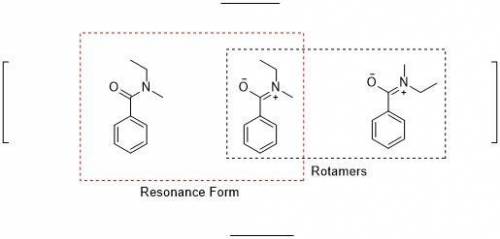
When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm–1. The 13C NMR and DEPT spectra are provided below. Draw the structure of the product as the resonance contributor lacking any formal charges.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/...
Questions in other subjects:

Mathematics, 09.12.2019 11:31

Social Studies, 09.12.2019 11:31


English, 09.12.2019 11:31

Mathematics, 09.12.2019 11:31




Mathematics, 09.12.2019 11:31