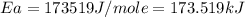For the gas phase isomerization of methyl cis-cinnamate, cis-C6H5CH=CHCOOCH3trans-C6H5CH=CHC OOCH3 the rate constant at 637 K is 1.88×10-4 s-1 and the rate constant at 679 K is 1.43×10-3 s-1. The activation energy for the gas phase isomerization of methyl cis-cinnamate is

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
For the gas phase isomerization of methyl cis-cinnamate, cis-C6H5CH=CHCOOCH3trans-C6H5CH=CHC OOCH3 t...
Questions in other subjects:




Health, 02.07.2019 12:40


Mathematics, 02.07.2019 12:40

Biology, 02.07.2019 12:40



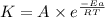
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0512/2904/6d953.png)
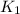 = rate constant at
= rate constant at 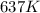 =
= 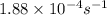
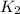 = rate constant at
= rate constant at 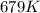 =
= 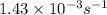
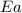 = activation energy for the reaction = ?
= activation energy for the reaction = ?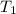 = initial temperature =
= initial temperature = 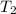 = final temperature =
= final temperature = ![\log (\frac{1.43\times 10^{-3}}{1.88\times 10^{-4}})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{637}-\frac{1}{679}]](/tpl/images/0512/2904/23370.png)
![0.88=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{637}-\frac{1}{679}]](/tpl/images/0512/2904/11d95.png)