

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 01:30, babygirl091502
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding. i
Answers: 1
You know the right answer?
Benzene has a formula of C6H6 and a vapor pressure of 96.4 torr at 298 K. Toluene has a formula of C...
Questions in other subjects:

Mathematics, 20.10.2019 07:30



Mathematics, 20.10.2019 07:30




Mathematics, 20.10.2019 07:30

Social Studies, 20.10.2019 07:30

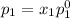 and
and 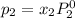
 = pressure in the pure state
= pressure in the pure state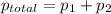




 = mole fraction of benzene =
= mole fraction of benzene =
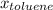 =mole fraction of toluene = (1-0.39) = 0.61
=mole fraction of toluene = (1-0.39) = 0.61




