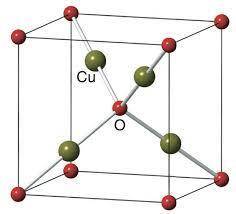
Cuprite, Cu2O, has a body-centered cubic unit cell of oxide anions with four copper cations in a tetrahedral arrangement around the body center oxide. Draw the unit cell in the empty cube. What is the coordination number and shape around the copper cations, and what is the charge on copper? Do the same analysis for the oxide anions.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Cuprite, Cu2O, has a body-centered cubic unit cell of oxide anions with four copper cations in a tet...
Questions in other subjects:


Mathematics, 04.08.2019 13:30

Mathematics, 04.08.2019 13:30

Health, 04.08.2019 13:30

History, 04.08.2019 13:30

Mathematics, 04.08.2019 13:30

Mathematics, 04.08.2019 13:30

Social Studies, 04.08.2019 13:30