Chemistry, 14.02.2020 23:27 bthakkar25
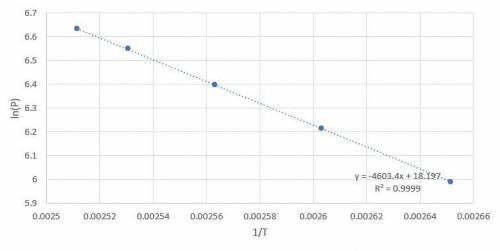
Octane is a liquid component of gasoline. Given the following vapor pressures of octane at various temperatures, estimate the boiling point of octane in Leadville, Colorado, where the atmospheric pressure is 496 mmHg.
a. 400 mmHg at 104oC
b. 500 mmHg at 111oC
c. 600 mmHg at 117oC
d. 700 mmHg at 122oC
e. 760 mmHg at 125oC

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 04:30, clairajogriggsk
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1
You know the right answer?
Octane is a liquid component of gasoline. Given the following vapor pressures of octane at various t...
Questions in other subjects:


Mathematics, 11.12.2019 06:31


English, 11.12.2019 06:31

History, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31



Mathematics, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31