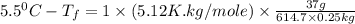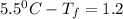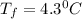Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
The freezing point of pure benzene is 5.5 o C. If 37 g TPP (tetraphenylporphryin, C44H30N4, MM = 614...
Questions in other subjects:








Chemistry, 10.10.2019 14:30

Mathematics, 10.10.2019 14:30

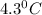

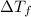 = change in freezing point =
= change in freezing point = 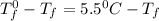
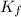 = freezing point constant = 5.12 K.kg/mole
= freezing point constant = 5.12 K.kg/mole