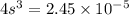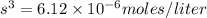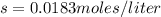Chemistry, 14.02.2020 21:21 romerok568
The K sp for barium fluoride, BaF2, is 2.45 × 10-5. What is the molar solubility of barium fluoride?

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 03:40, ElegantEmerald
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
The K sp for barium fluoride, BaF2, is 2.45 × 10-5. What is the molar solubility of barium fluoride?...
Questions in other subjects:


Biology, 16.10.2020 05:01



Mathematics, 16.10.2020 05:01


Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

English, 16.10.2020 05:01

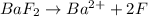
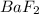 gives 2 moles of
gives 2 moles of 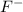 and 1 mole of
and 1 mole of 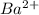
![K_sp=[Ba^{2+}][F^{-}]^2](/tpl/images/0511/8336/a05b2.png)
![2.45\times 10^{-5}=[s][2s]^2](/tpl/images/0511/8336/a4b76.png)