

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
The gas phase reaction 2 N2O5(g) → 4 NO2(g) + O2(g) has an activation energy of 103 kJ/mol, and the...
Questions in other subjects:










Mathematics, 18.02.2020 20:06



![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0511/7907/6d953.png)
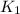 = rate constant at 266 K =
= rate constant at 266 K = 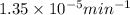
 = rate constant at 296 K = ?
= rate constant at 296 K = ? = activation energy for the reaction = 103 kJ/mol = 103000 J/mol
= activation energy for the reaction = 103 kJ/mol = 103000 J/mol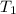 = initial temperature = 266 K
= initial temperature = 266 K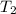 = final temperature = 296 K
= final temperature = 296 K![\log (\frac{K_2}{1.35\times 10^{-5}})=\frac{103000}{2.303\times 8.314J/mole.K}[\frac{1}{266}-\frac{1}{296}]](/tpl/images/0511/7907/d42c0.png)




