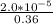Chemistry, 14.02.2020 19:33 camirialchambers17
The common-ion effect can be applied to a solution of lead thiocyanate when potassium thiocyanate is added. Since there is a common ion, the cyanate ion, the molar solubility of lead thiocyanate goes down in the presence of potassium thiocyanate. Calculate the molar solubility of lead thiocyanate in 0.600 M KSCN.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
The common-ion effect can be applied to a solution of lead thiocyanate when potassium thiocyanate is...
Questions in other subjects:


Mathematics, 23.06.2019 11:40





Mathematics, 23.06.2019 11:40



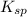 of Pb(SCN)₂ is known to be 2.0 × 10⁻⁵
of Pb(SCN)₂ is known to be 2.0 × 10⁻⁵