
Chemistry, 14.02.2020 19:19 mackdoolittle1
What is the concentration of a solution of (a) KOH for which the pH is 11.89; (b) Ca(OH)2 for which the pH is 11.68

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:00, BeeShyanne
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 09:00, sammypaige08
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2

Chemistry, 23.06.2019 10:50, uh8hardiek
Mach the labels with the symbols on the weather map
Answers: 2
You know the right answer?
What is the concentration of a solution of (a) KOH for which the pH is 11.89; (b) Ca(OH)2 for which...
Questions in other subjects:








Mathematics, 03.03.2020 01:36


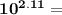 [OH⁻] = 7.76×10⁻³ M
[OH⁻] = 7.76×10⁻³ M 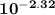 = [OH⁻] = 4.78×10⁻³ M
= [OH⁻] = 4.78×10⁻³ M
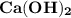 is 2:1.
is 2:1.


