
Chemistry, 14.02.2020 19:16 lindsaypre0644
What is the maximum amount of silver (in grams) that can be plated out of 4.6 LL of an AgNO3AgNO3 solution containing 3.3 %% AgAg by mass? Assume that the density of the solution is 1.01 g/mLg/mL.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:50, datboyjulio21
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
What is the maximum amount of silver (in grams) that can be plated out of 4.6 LL of an AgNO3AgNO3 so...
Questions in other subjects:

Geography, 28.01.2020 03:31



Mathematics, 28.01.2020 03:31


History, 28.01.2020 03:31


English, 28.01.2020 03:31

Arts, 28.01.2020 03:31

Mathematics, 28.01.2020 03:31

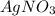 contains 3.3% Ag
contains 3.3% Ag

