
Chemistry, 14.02.2020 18:27 kylucienne
The popularity of orange juice, especially as a breakfast drink, makes it an important factor in the economy of orange-growing regions. Marketed juice has either gone through a process in which it was concentrated, or it may be a not-from-concentrate juice. Frozen concentrated juice is reconstituted before consumption. Although concentrated juices are less popular in the United States than at one time, they still have a major segment of the market. The approaches to concentrating orange juice include evaporation, freeze concentration, and reverse osmosis. Here we examine the evaporation process by focusing only on two constituents in the juice: solids and water. Fresh orange juice contains approximately 10.0 wt% solids (sugar, citric acid and other indigenous ingredients) and frozen concentrate contains approximately 42.0 wt% solids. The frozen concentrate is obtained by evaporating water from the fresh juice to produce a mixture that is approximately 57.0 wt% solids. However, so that the flavor of the concentrate will closely approximate that of fresh juice, the concentrate from the evaporator is blended with fresh orange juice (and other minor additives) to produce a final concentrate that is approximately 42.0 wt% solids. Assume a basis of 300.0 kg of fresh juice fed to the process.
Calculate the amount of product (42% concentrate) produced per 100kg fresh juice fed to theprocess and the fraction of the feed that bypasses the evaporator.

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
The popularity of orange juice, especially as a breakfast drink, makes it an important factor in the...
Questions in other subjects:

Mathematics, 16.02.2021 04:00

Mathematics, 16.02.2021 04:00

History, 16.02.2021 04:00

Mathematics, 16.02.2021 04:00

Mathematics, 16.02.2021 04:00




Arts, 16.02.2021 04:00

English, 16.02.2021 04:00

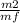 =
= 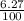 = 0.0627
= 0.0627

