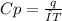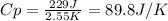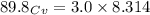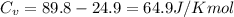Chemistry, 14.02.2020 17:25 postorivofarms
When 229 J of energy is supplied as heat to 3.0 mol of nitrogen N2(g) at constant pressure, the temperature of the sample increases by 2.55 K. Assuming that under these conditions nitrogen behaves as an ideal gas, what is the value of the molar heat capacity at constant volume of N2(g)?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 05:30, saleenhernandez83
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
When 229 J of energy is supplied as heat to 3.0 mol of nitrogen N2(g) at constant pressure, the temp...
Questions in other subjects:


Geography, 27.07.2019 13:20



Social Studies, 27.07.2019 13:20

Mathematics, 27.07.2019 13:20

Mathematics, 27.07.2019 13:20

Computers and Technology, 27.07.2019 13:20

Mathematics, 27.07.2019 13:20

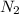 is 64.9 J/Kmol
is 64.9 J/Kmol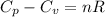
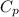 = heat capacity at constant pressure
= heat capacity at constant pressure 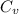 = heat capacity at constant volume
= heat capacity at constant volume