. Like any equilibrium constant, Kw, changes with temperature. (a) Given that autoionization is endothermic, how does Kw change with rising T? Explain with a reaction that includes heat as a reactant or product. (b) In many medical applications, the value of Kw at 37°C (body T) may be more appropriate than the value at 25°C, 1.0x10-14. The pH of pure at 37°C is 6.80. Calculate Kw, pOH and [OH-] at this T.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 23.06.2019 12:30, 1Angel2Got3Brains
Is the genie in the bottle experiment a physical or chemical change/reaction?
Answers: 1

Chemistry, 23.06.2019 21:00, alyssamaize
Using the periodic table choose the more reactive nonmetal s or as
Answers: 1
You know the right answer?
. Like any equilibrium constant, Kw, changes with temperature. (a) Given that autoionization is endo...
Questions in other subjects:

Mathematics, 01.02.2021 23:30





Biology, 01.02.2021 23:30


History, 01.02.2021 23:30

Chemistry, 01.02.2021 23:30

History, 01.02.2021 23:30

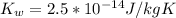
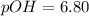
![[OH^{-}] =1.6*10^{-7} M](/tpl/images/0511/5212/a2f6d.png)