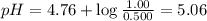Chemistry, 14.02.2020 03:28 andydiaz1227
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 Group of answer choices 4.47 5.06 4.77 0.3 Flag this Question

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
What is the pH of a solution that is 0.500 M in acetic acid and 1.00 M in CH3COONa? Ka = 1.75*10-5 G...
Questions in other subjects:

English, 01.09.2020 20:01


Mathematics, 01.09.2020 20:01

English, 01.09.2020 20:01

Social Studies, 01.09.2020 20:01

Mathematics, 01.09.2020 20:01

Physics, 01.09.2020 20:01



History, 01.09.2020 20:01