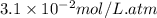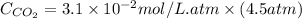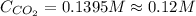Chemistry, 14.02.2020 02:27 cookie42087
Calculate the concentration of CO2 in water at 25 degrees Celsius when the pressure of CO2 over the solution is 4.5 atm. At 25 degrees Celsius, the Henry's law constant for CO2 in water is 3.1 * 10^-2 mol/L*atm.
? M

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
Calculate the concentration of CO2 in water at 25 degrees Celsius when the pressure of CO2 over the...
Questions in other subjects:




Mathematics, 21.12.2021 07:40

Social Studies, 21.12.2021 07:40





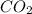 is, 0.12 M
is, 0.12 M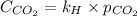
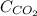 = concentration of
= concentration of 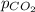 = partial pressure of
= partial pressure of 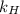 = Henry's law constant =
= Henry's law constant =