
Chemistry, 14.02.2020 01:41 katwright1124
Consider the following system at equilibrium where H° = -111 kJ, and Kc = 0.159 , at 723 K: N2 (g) + 3 H2 (g) 2 NH3 (g)
If the TEMPERATURE on the equilibrium system is suddenly decreased :
The value of Kc A. Increases B. Decreases C. Remains the same
The value of QcA. Is greater than Kc B. Is equal to Kc C. Is less than Kc
The reaction must: A. Run in the forward direction to restablish equilibrium. B. Run in the reverse direction to restablish equilibrium. C. Remain the same. Already at equilibrium.
The concentration of H2 will: A. Increase. B. Decrease. C. Remain the same.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
Consider the following system at equilibrium where H° = -111 kJ, and Kc = 0.159 , at 723 K: N2 (g) +...
Questions in other subjects:

Mathematics, 27.09.2021 14:00

History, 27.09.2021 14:00


History, 27.09.2021 14:00

Chemistry, 27.09.2021 14:00

History, 27.09.2021 14:00




English, 27.09.2021 14:00

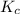 will increase.
will increase.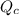 will decrease.
will decrease.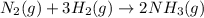
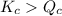 ( Move in froward direction )
( Move in froward direction )

