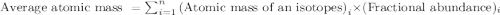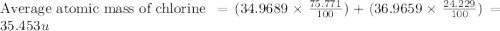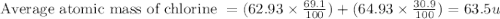Chemistry, 13.02.2020 23:28 beccadrums
Chlorine has two isotopes. Chlorine-35 has an actual mass of 34.9689 u and chlorine-37 has a mass of 36.9659 u. In any sample of chlorine atoms, 75.771% will be chlorine-35 and 24.229% will be chlorine 37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1


Chemistry, 23.06.2019 03:00, EllaLovesAnime
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
Chlorine has two isotopes. Chlorine-35 has an actual mass of 34.9689 u and chlorine-37 has a mass of...
Questions in other subjects:



History, 16.10.2020 06:01




Mathematics, 16.10.2020 06:01