Chemistry, 13.02.2020 22:26 GreenHerbz206
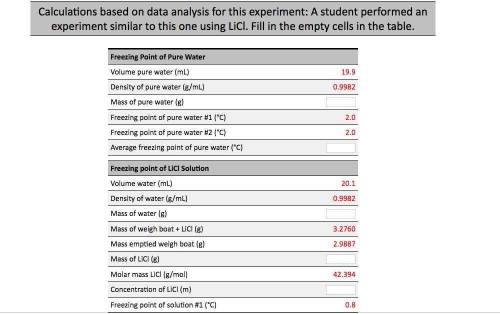
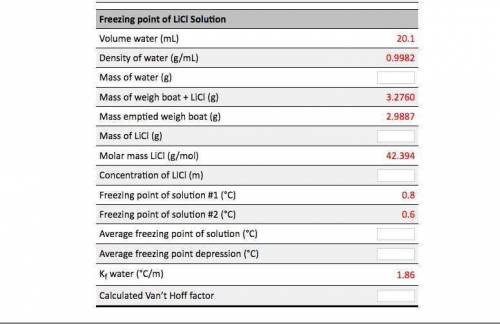
Calculations based on data analysis for this experiment: A student performed an experiment similar to this one using LiCl. Fill in the empty cells in the table. Freezing Point of Pure Water Volume pure water (mL) 19.9 Density of pure water (g/mL) 0.9982 Mass of pure water (8) Freezing point of pure water #1 (°C) Freezing point of pure water #2 (°C) Average freezing point of pure water (°C) Freezing point of Licl Solution 20.1 Volume water (mL) Density of water (g/mL) Mass of water (g) 0.9982 3.2760 Mass of weigh boat + LiCl (g) Mass emptied weigh boat (g) 2.9887 Mass of LiCl (8) 42.394 Molar mass LiCl (g/mol) Concentration of LiCl (m) Freezing point of solution #1 (°C) 0.8 Freezing point of Licl Solution Volume water (ml) 20.1 0.9982 3.2760 2.9887 = 42.394 Density of water (g/mL) Mass of water (g) Mass of weigh boat + LICI (g) Mass emptied weigh boat (g) Mass of LiCl (g) Molar mass Licl (g/mol) Concentration of LiCl (m) Freezing point of solution #1 (°C) Freezing point of solution #2 (°C) Average freezing point of solution (°C) Average freezing point depression (°C) Kf water (°C/m) Calculated Van't Hoff factor 0.8 1.86

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 20:40, ohgeezy
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
Calculations based on data analysis for this experiment: A student performed an experiment similar t...
Questions in other subjects:

Mathematics, 20.05.2020 07:00



English, 20.05.2020 07:00

Mathematics, 20.05.2020 07:00



History, 20.05.2020 07:01

Mathematics, 20.05.2020 07:01

Social Studies, 20.05.2020 07:01