Chemistry, 13.02.2020 22:22 haileyrae187
One mole of an ideal gas is contained in a cylinder with a movable piston. The temperature is constant at 778C. Weights are removed suddenly from the piston to give the following sequence of three pressures: a. P1 5 5.00 atm (initial state) b. P2 5 2.24 atm c. P3 5 1.00 atm (final state)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, alexisbcatlett14
Which statement can best be concluded from the ideal gas law?
Answers: 2



Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
One mole of an ideal gas is contained in a cylinder with a movable piston. The temperature is consta...
Questions in other subjects:

Biology, 18.12.2019 15:31





History, 18.12.2019 15:31

Social Studies, 18.12.2019 15:31


History, 18.12.2019 15:31

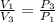
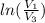 =
= 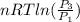
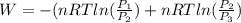 or
or 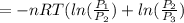
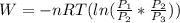 =
= 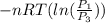 therefore the equation is the same for calculating directly from the initial pressure P₁, to the final pressure P₃
therefore the equation is the same for calculating directly from the initial pressure P₁, to the final pressure P₃