
Chemistry, 13.02.2020 22:21 viktoria1198zz
Assume that the reaction for the formation of gaseous hydrogen fluoride from hydrogen and fluorine has an equilibrium constant of 1.15 X 102 at a certain temperature. In a particular experiment, 3.00 mole of each component was added to a 1.50 L flask. Calculate the equilibrium concentrations of all species.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Assume that the reaction for the formation of gaseous hydrogen fluoride from hydrogen and fluorine h...
Questions in other subjects:



Mathematics, 20.07.2019 18:00

Biology, 20.07.2019 18:00





History, 20.07.2019 18:00

![[H_2]= 0.314 M](/tpl/images/0510/5228/1fffd.png)
![[F_2]=0.314 M](/tpl/images/0510/5228/5de3f.png)
![[HF]=3.372 M](/tpl/images/0510/5228/8e291.png)
![[H_2]=\frac{3.00 mol}{1.5 L}=2.0 M](/tpl/images/0510/5228/f9117.png)
![[F_2]=\frac{3.00 mol}{1.5 L}=2.0 M](/tpl/images/0510/5228/b656e.png)
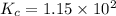
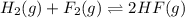
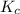 is given by :
is given by :![K_c=\frac{[HF]^2}{[H_2][F_2]}](/tpl/images/0510/5228/a2854.png)
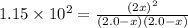
![[H_2]=(2.0 -1.686)M = 0.314 M](/tpl/images/0510/5228/64b44.png)
![[F_2]=(2.0 -1.686)M = 0.314 M](/tpl/images/0510/5228/f4a49.png)
![[HF]=(2\times 1.686)M = 3.372 M](/tpl/images/0510/5228/dc9b5.png)


