
Chemistry, 13.02.2020 21:43 PONBallfordM89
What is the molarity and molality of a solution that is 10.00 % by mass potassium hydrogen carbonate (KHCO3, 100.11 g/mol) and has a density of 1.0650 g/mL

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1

Chemistry, 23.06.2019 09:20, goldwinner300
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2

Chemistry, 23.06.2019 09:30, kleathers97
Hey, could someone me answer this? much appreciated!
Answers: 1
You know the right answer?
What is the molarity and molality of a solution that is 10.00 % by mass potassium hydrogen carbonate...
Questions in other subjects:

English, 31.08.2019 19:30



Mathematics, 31.08.2019 19:30






Spanish, 31.08.2019 19:30

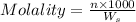
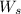 = weight of solvent in g
= weight of solvent in g 
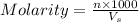
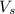 = volume of solution in ml
= volume of solution in ml



