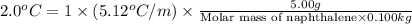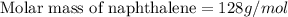Chemistry, 13.02.2020 21:27 jnsoccerboy3121
Calculate the molar mass of naphthalene, the organic compound in mothballs, if a solution prepared by dissolving 5.00 g of naphthalene in exactly 100 g of benzene has a freezing point 2.0°C below that of pure benzene. (Kf of benzene is 5.12°C/m.)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
Calculate the molar mass of naphthalene, the organic compound in mothballs, if a solution prepared b...
Questions in other subjects:

Mathematics, 07.12.2020 08:00

Spanish, 07.12.2020 08:00

Mathematics, 07.12.2020 08:00


Mathematics, 07.12.2020 08:00

Mathematics, 07.12.2020 08:00



Mathematics, 07.12.2020 08:00

Biology, 07.12.2020 08:00

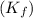 for benzene =
for benzene = 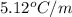
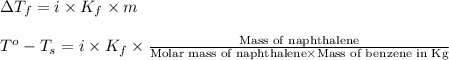
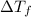 = change in freezing point =
= change in freezing point = 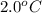
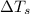 = freezing point of solution
= freezing point of solution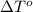 = freezing point of benzene
= freezing point of benzene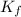 = freezing point constant for benzene =
= freezing point constant for benzene =