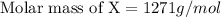Chemistry, 13.02.2020 18:46 kingdrex4772
When 19g of a certain molecular compound X are dissolved in of benzonitrile , the freezing point of the solution is measured to be . Calculate the molar mass of X.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, angelicar1160
How do scientists think that gravity affected the formation of our solar system?
Answers: 1



Chemistry, 23.06.2019 13:30, michelle8978
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide
Answers: 1
You know the right answer?
When 19g of a certain molecular compound X are dissolved in of benzonitrile , the freezing point of...
Questions in other subjects:

Mathematics, 14.11.2019 01:31

Geography, 14.11.2019 01:31

Mathematics, 14.11.2019 01:31

History, 14.11.2019 01:31


Mathematics, 14.11.2019 01:31

Mathematics, 14.11.2019 01:31

Chemistry, 14.11.2019 01:31

Mathematics, 14.11.2019 01:31

Mathematics, 14.11.2019 01:31

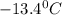 . Calculate the molar mass of X. Freezing point of pure benzonitrile =
. Calculate the molar mass of X. Freezing point of pure benzonitrile = 

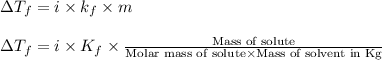
 = change in freezing point =
= change in freezing point = 
 = freezing point constant = 5.35 K.kg/mole
= freezing point constant = 5.35 K.kg/mole