

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Using the systematic approach for equilibrium problems, calculate the pH of 0.05 M HOCl. Ka= 3.0*10-...
Questions in other subjects:




Mathematics, 27.02.2020 22:42


Biology, 27.02.2020 22:43




![Ka=\frac{[ClO-]*[H+]}{[HClO]}=\frac{x*x}{0.05-x}=3x10^{-8}](/tpl/images/0510/1394/ee229.png)
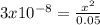
![x=3.87x10^{-5}=[H+]=[ClO-]](/tpl/images/0510/1394/d0c35.png)
![pH=-log[H+]=-log[3.87x10^{-5}]=4.41](/tpl/images/0510/1394/b0e39.png)


