Chemistry, 13.02.2020 18:40 llamasking
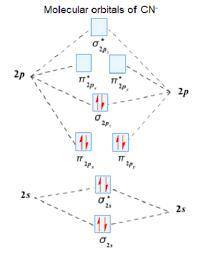
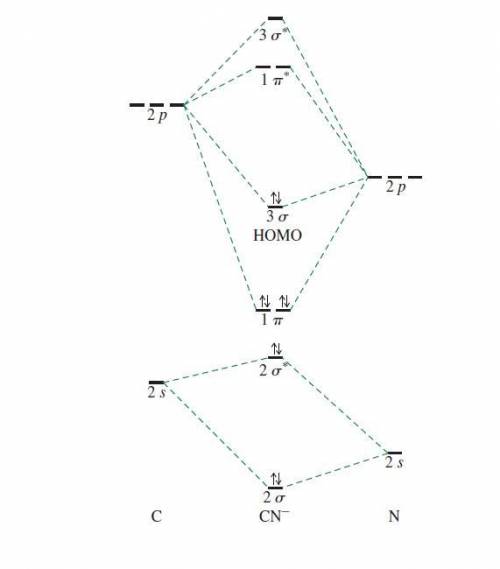
Using the molecular orbital model, write electron configurations for the following diatomic species and calculate the bond orders. Which ones are paramagnetic? Place the species in order of increasing bond length and bond energy.
a. CN +
b. CN
c. CN -

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1


Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Using the molecular orbital model, write electron configurations for the following diatomic species...
Questions in other subjects:

Mathematics, 06.06.2020 00:00

Mathematics, 06.06.2020 00:00

Mathematics, 06.06.2020 00:00


Mathematics, 06.06.2020 00:00


Biology, 06.06.2020 00:00