
A certain substance X has a normal freezing point of -10.1 degree C and a molal freezing point depression constant K_f = 5.32 degree C middot kg middot mol^-1. Calculate the freezing point of a solution made of 29.82 g of urea ((NH_2)_CO) dissolved in 500. g of X. Be sure your answer has the correct number of significant digits.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2


Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
A certain substance X has a normal freezing point of -10.1 degree C and a molal freezing point depre...
Questions in other subjects:

Geography, 23.04.2020 01:02




Mathematics, 23.04.2020 01:02

Biology, 23.04.2020 01:02

History, 23.04.2020 01:02

Mathematics, 23.04.2020 01:03


Mathematics, 23.04.2020 01:03


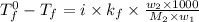
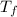 = boiling point of solution = ?
= boiling point of solution = ? = boiling point of solvent (X) =
= boiling point of solvent (X) = 
 = freezing point constant =
= freezing point constant = 
 = molar mass of solute (urea) = 60 g/mol
= molar mass of solute (urea) = 60 g/mol




