
You considered the properties of two acid-base indicators, phenolphthalein and methyl orange. Many indicators are weak acids in water and establish the equilibrium: HIn(aq)(Color 1)+ H2O(l) → H3O (aq) + In-(aq)(Color 2). Indicators change color depending on whether they are in a protonated (HIn) or unprotonated (In-) form. What is the equilibrium expression for the phenolphthalein indicator in water and what colors are the protonated and unprotonated forms of the indicator?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, mere33
Janel’s class studied properties of matter and how matter can change. janel decided she would do an experiment mixing baking soda and vinegar. question: describe the properties of baking soda and vinegar, and explain the changes that janel should see when she mixes the two types of matter. •first, identify the physical state of matter of baking soda. describe another property of baking soda. •next, identify the physical state of matter of vinegar. describe another property of vinegar. •then, explain what janel should see when she mixes the baking soda and vinegar. •describe the states of matter of the new materials that are formed. •explain how janel can be certain a change has occurred. me
Answers: 3


Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1
You know the right answer?
You considered the properties of two acid-base indicators, phenolphthalein and methyl orange. Many i...
Questions in other subjects:

Chemistry, 23.07.2019 10:20







Geography, 23.07.2019 10:20


Health, 23.07.2019 10:20

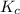
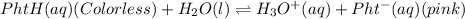
![K_c=\frac{[H_3O^+][Pht^-]}{[PhtH]}](/tpl/images/0509/9728/68136.png)


