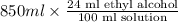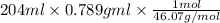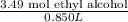Chemistry, 13.02.2020 05:54 haileyparrill703
A 850.0 mL bottle of Listerine is of a 24 % (v/v) ethyl alcohol. If the density of ethyl alcohol is 0.789 g/mL and the molar mass is 46.07 g/mol, calculate the molarity of ethyl alcohol in Listerine.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
A 850.0 mL bottle of Listerine is of a 24 % (v/v) ethyl alcohol. If the density of ethyl alcohol is...
Questions in other subjects:

Biology, 11.10.2020 08:01



History, 11.10.2020 08:01

Mathematics, 11.10.2020 08:01


Mathematics, 11.10.2020 08:01


Mathematics, 11.10.2020 08:01

Biology, 11.10.2020 08:01