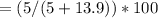Chemistry, 13.02.2020 03:25 taylordalton93
A solution contains 5.00 5.00 g of solute in 13.9 13.9 g of solvent. What is the mass percent of the solute in the solution?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 23.06.2019 07:40, Aaron5795
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
A solution contains 5.00 5.00 g of solute in 13.9 13.9 g of solvent. What is the mass percent of the...
Questions in other subjects:


Arts, 08.07.2019 18:30

Social Studies, 08.07.2019 18:30

Mathematics, 08.07.2019 18:30


Social Studies, 08.07.2019 18:30



Chemistry, 08.07.2019 18:30