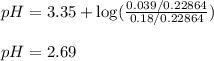Problem PageQuestion An analytical chemist is titrating of a solution of nitrous acid with a solution of . The of nitrous acid is . Calculate the pH of the acid solution after the chemist has added of the solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to decimal places.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Problem PageQuestion An analytical chemist is titrating of a solution of nitrous acid with a solutio...
Questions in other subjects:



Mathematics, 07.03.2020 03:10


Mathematics, 07.03.2020 03:10





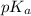 of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 46.44 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to 2 decimal places.
of nitrous acid is 3.35. Calculate the pH of the acid solution after the chemist has added 46.44 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of solution added. Round your answer to 2 decimal places.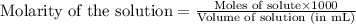
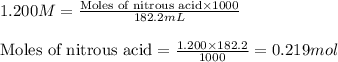


![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0509/3412/e4eea.png)
![pH=pK_a+\log(\frac{[KNO_2]}{[HNO_2]})](/tpl/images/0509/3412/fa3ae.png)
![[KNO_2]=\frac{0.039}{0.22864}](/tpl/images/0509/3412/7262b.png)
![[HNO_2]=\frac{0.18}{0.22864}](/tpl/images/0509/3412/eee2b.png)