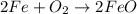A student reacts steel wool with oxygen by touching the fibers with a 9 volt battery. They begin
with 7.93 g Fe and measure the mass of the final product at 9.50 g.
The balanced equation for the reaction is:
2 Fe + 02 --> 2 FeO
A) The student claims that the percent yield of the reaction was 93.1 %. Support or reject their
claim including a calculation of percent yield as part of your evidence.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 23:00, genyjoannerubiera
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
A student reacts steel wool with oxygen by touching the fibers with a 9 volt battery. They begin
Questions in other subjects:



English, 19.02.2020 04:27

Mathematics, 19.02.2020 04:27




Biology, 19.02.2020 04:28