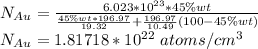Chemistry, 13.02.2020 00:38 seannalove4148
Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic centimeter for a silver-gold alloy that contains 45 wt% Au and 55 wt% Ag. The densities of pure gold and silver are 19.32 and 10.49 g/cm3, respectively. The atomic weight of Au is 196.97 g/mol.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Gold forms a substitutional solid solution with silver. Compute the number of gold atoms per cubic c...
Questions in other subjects:


English, 24.11.2019 07:31




Computers and Technology, 24.11.2019 07:31




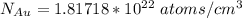
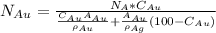
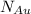 are number of gold atoms.
are number of gold atoms.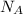 is Avogadro Number.
is Avogadro Number.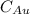 is the amount of gold.
is the amount of gold.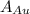 is the atomic weight of gold.
is the atomic weight of gold.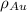 is the density of gold.
is the density of gold.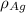 is the density of silver.
is the density of silver.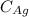 is the amount of silver.
is the amount of silver.