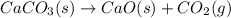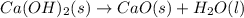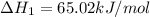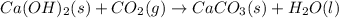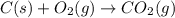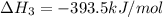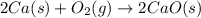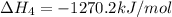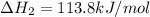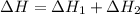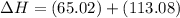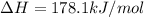Chemistry, 12.02.2020 23:10 makayladurham19
Determine the enthalpy change for the decomposition of calcium carbonate. CaCO3 (s) --> CaO (s) + CO2 (g) given the thermochemical equations below:
Ca(OH)2 (s) --> CaO (s) + H2O (l) enthalpy reaction = 65.2 kJ/mol
Ca(OH)2 (s) + CO2(g) --> CaCO3 (s) + H2O (l) enthalpy reaction = -113.8 kJ/mol
C(s) + O2 (g) --> CO2 (g) enthalpy of reation = -393.5 kJ/mol
2Ca(s) + O2(g) --> 2 CaO (s) enthalpy of reaction = -1270.2 kJ/mol
a. 1711.7 kJ/mol rxn
b. 441 kJ/mol rxn
c. 179 kJ/mol rxn
d. 48 kJ/mol rxn
e. 345.5 kJ. mol rxn

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1
You know the right answer?
Determine the enthalpy change for the decomposition of calcium carbonate. CaCO3 (s) --> CaO (s) +...
Questions in other subjects:



Mathematics, 30.06.2019 10:30

Mathematics, 30.06.2019 10:30

Biology, 30.06.2019 10:30




Mathematics, 30.06.2019 10:30