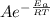Chemistry, 12.02.2020 05:30 dededese2403
Which of the following statements is TRUE?a. The rate constant does not depend on the activation energy for a reaction where the products are lower in energy than the reactants. b. The addition of a homogeneous catalyst does not change the activation energy of a given reaction. c. Rate constants are temperature dependent. d. None of the above is true. e. A catalyst raises the activation energy of a reaction.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Which of the following statements is TRUE?a. The rate constant does not depend on the activation ene...
Questions in other subjects:


English, 03.03.2021 04:40


Mathematics, 03.03.2021 04:40

English, 03.03.2021 04:40


Mathematics, 03.03.2021 04:40

Social Studies, 03.03.2021 04:40


Chemistry, 03.03.2021 04:40