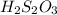A laboratory analysis of a 100 g sample finds it is composed of 1.8 g hydrogen, 56.1 g sulfur, and 42.1 g oxygen. What is its empirical formula? Give your answer in the form H#S#O#, where the number following the element’s symbol corresponds to the subscript in the formula. (Don’t include a 1 subscript explicitly.) For example, the formula CHO would be entered as CH2O.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
A laboratory analysis of a 100 g sample finds it is composed of 1.8 g hydrogen, 56.1 g sulfur, and 4...
Questions in other subjects:

Mathematics, 09.02.2021 22:00




English, 09.02.2021 22:00

Advanced Placement (AP), 09.02.2021 22:00



English, 09.02.2021 22:00

Chemistry, 09.02.2021 22:00