Chemistry, 12.02.2020 04:48 montgomeryaevans
A pure radioactive substance has a mass of 30 micrograms but had a mass of 240 micrograms 48 years ago. Assuming that the only changes in mass are due to radioactive decay, the half-life of the substance is years. Please choose the correct answer from the following choices, and then select the submit answer button.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, penny3109
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1


Chemistry, 21.06.2019 23:30, tylerineedhelp
Ihat will happen if i added baking soda to vinegar
Answers: 2

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2
You know the right answer?
A pure radioactive substance has a mass of 30 micrograms but had a mass of 240 micrograms 48 years a...
Questions in other subjects:








Mathematics, 02.08.2019 04:40

Mathematics, 02.08.2019 04:40

Mathematics, 02.08.2019 04:40

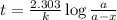
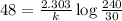
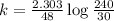
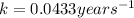
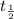 of reaction
of reaction