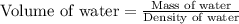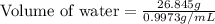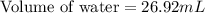Chemistry, 12.02.2020 04:41 carlosgc19
A student obtained a clean, dry, glass-stoppered flask. He weighed the flask stopper and found the total mass to be 32.634g. He then filled the flask with water, weighed again, and obtained a mass of 59.479g. At the temperature of the water, he found that its density was 0.9973 g/mL. a.) What was the mass of the water? (show work)b.) What was the volume of the water? (Show work)c.) What was the volume of the flask? (show work)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
A student obtained a clean, dry, glass-stoppered flask. He weighed the flask stopper and found the t...
Questions in other subjects:



History, 06.10.2019 09:30



History, 06.10.2019 09:30

Mathematics, 06.10.2019 09:30