
Chemistry, 12.02.2020 04:19 monsterenergy19
Check all the true statements concerning two identical containers, one with helium gas in it and one with xenon gas. Choose one or more:
a. When the temperature of either sample of gas increases, the root-mean-square speed increases.
b. If both gases are at the same temperature, they have the same average kinetic energy.
c. When the temperature of either sample of gas increases, the number of particles with average kinetic energy increases.
d. If both of the containers are at the same temperature, they will have the same root-mean-square speed.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1


Chemistry, 22.06.2019 02:30, jrjimenez
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2
You know the right answer?
Check all the true statements concerning two identical containers, one with helium gas in it and one...
Questions in other subjects:




History, 18.03.2020 09:25

Mathematics, 18.03.2020 09:25

Mathematics, 18.03.2020 09:26

Mathematics, 18.03.2020 09:26

Mathematics, 18.03.2020 09:26


Mathematics, 18.03.2020 09:27


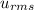 = root mean square speed
= root mean square speed we see that the kinetic energy is directly proportional to its Kelvin temperature and hence b. is true
we see that the kinetic energy is directly proportional to its Kelvin temperature and hence b. is true

