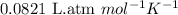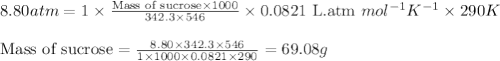Chemistry, 12.02.2020 03:24 cuppykittyy
What mass of sucrose (C12H22O11) should be combined with 546 g of water to make a solution with an osmotic pressure of 8.80 atm at 290 K ? (Assume the density of the solution to be equal to the density of the solvent.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 22:30, jaylenmiller437
The diagram shows the relationship between scientific disciplines. the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a. physics b. biology c. chemistry d. metallurgy
Answers: 2
You know the right answer?
What mass of sucrose (C12H22O11) should be combined with 546 g of water to make a solution with an o...
Questions in other subjects:

Biology, 09.08.2019 23:20




Mathematics, 09.08.2019 23:20


Mathematics, 09.08.2019 23:20

History, 09.08.2019 23:20


Mathematics, 09.08.2019 23:20

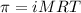
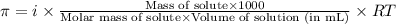
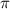 = osmotic pressure of the solution = 8.80 atm
= osmotic pressure of the solution = 8.80 atm