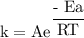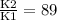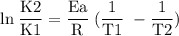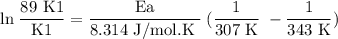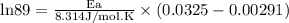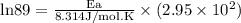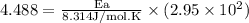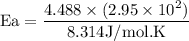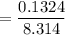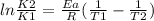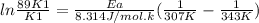Chemistry, 12.02.2020 03:19 CoolRahim9090
What is the activation energy (in kJ/mol) of a reaction whose rate constant increases by a factor of 89 upon increasing the temperature from 307 K to 343 K? R = 8.314 J/(mol • K). Only enter the numerical value as an integer in the answer box below. Do NOT type in the unit (kJ/mol).

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
What is the activation energy (in kJ/mol) of a reaction whose rate constant increases by a factor of...
Questions in other subjects:



Mathematics, 09.09.2019 07:20



Mathematics, 09.09.2019 07:20

History, 09.09.2019 07:20


History, 09.09.2019 07:20

Chemistry, 09.09.2019 07:20