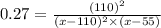Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOC...

Chemistry, 12.02.2020 02:45 ghaithalhamdani
Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOCl(g)
Kp=0.27 at 700 K
A reaction mixture initially contains equal partial pressures of NO and Cl2. At equilibrium, the partial pressure of NOCl was measured to be 110 torr. What were the initial partial pressures of NO and Cl2?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
Questions in other subjects:










 and
and 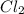 are 134 torr each.
are 134 torr each.
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0507/8534/56950.png)