
Chemistry, 12.02.2020 02:25 shelbylynn737
The [α] of pure quinine, an antimalarial drug, is −165. If a solution contains 86% quinine and 14% of its enantiomer (ee = 72%), what is [α] for the solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
The [α] of pure quinine, an antimalarial drug, is −165. If a solution contains 86% quinine and 14% o...
Questions in other subjects:

Mathematics, 18.01.2021 07:50

English, 18.01.2021 07:50



Mathematics, 18.01.2021 07:50

English, 18.01.2021 07:50

Biology, 18.01.2021 07:50

Mathematics, 18.01.2021 07:50




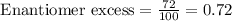
![[\alpha]=\text{Enantiomer excess}\times [\alpha]_{Pure}](/tpl/images/0507/8364/235fc.png)
![[\alpha]=0.72\times -165](/tpl/images/0507/8364/0bb55.png)
![[\alpha]=-118.8](/tpl/images/0507/8364/055ac.png)


