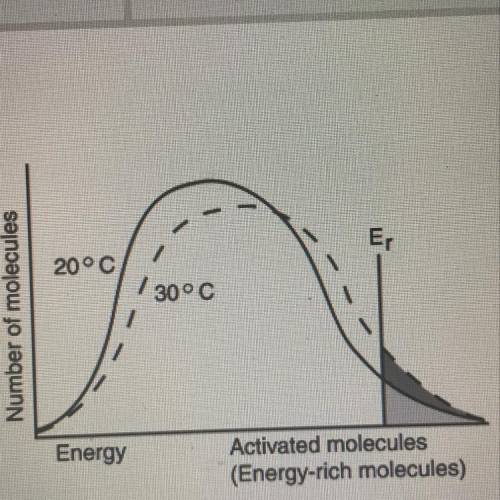
From the diagram, when the temperatures of reactants are raised from 20°C to 30°C:
The average...


Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
Questions in other subjects:

Chemistry, 05.02.2020 02:47

History, 05.02.2020 02:47

Arts, 05.02.2020 02:47



Social Studies, 05.02.2020 02:47


Social Studies, 05.02.2020 02:47


Arts, 05.02.2020 02:47