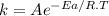The rate constant for a reaction is measured as a function of time. A plot is created by graphing ln(k) on the y-axis and 1/T on the x-axis, and a best fit line with a slope of -10,473 K is obtained. Based on this data what is the activation energy of this reaction (in kJ/mol)?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
The rate constant for a reaction is measured as a function of time. A plot is created by graphing ln...
Questions in other subjects:


Mathematics, 04.02.2021 15:50


Mathematics, 04.02.2021 15:50

Mathematics, 04.02.2021 15:50


Chemistry, 04.02.2021 15:50

Mathematics, 04.02.2021 15:50