Chemistry, 11.02.2020 22:25 xthom001ow7go3
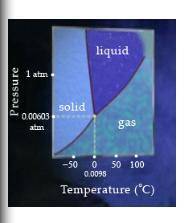
The triple point of water is 0.0098%u2218C at 0.00603 atm (4.58 torr). At the triple point, ice, water, and water vapor exist in equilibrium with each other.
Complete the following sentences to identify the process that ice, water, or water vapor may undergo if either the temperature or the pressure is increased.
Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.
FREEZE 1. If ice is heated at a constant pressure of 0.00512 atm, it will.
CONDENSE 2. If ice is heated at a constant pressure of 1 atm, it will.
MELT 3. If the pressure of water vapor is increased at a constant pressure of 100 degrees Celsius, it will.
SUBLIME 4. If the pressure of water vapor is increased at a constant pressure of -50 degrees Celsius, it will__.
VAPORIZE
DEPOSIT

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1


Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
The triple point of water is 0.0098%u2218C at 0.00603 atm (4.58 torr). At the triple point, ice, wat...
Questions in other subjects:



SAT, 01.10.2019 06:30

Mathematics, 01.10.2019 06:30

Computers and Technology, 01.10.2019 06:30




Mathematics, 01.10.2019 06:30